IBDoc® is present in the market for 6 years now and its efficiency and usefulness has been described many times by different research teams all over the world (1, 2, 3). All of these papers are evaluating IBDoc® as a stand-alone product but IBDoc® has the possibility to be integrated in a 3rd party application, thanks to its Application Programming Interface (API). In disease management of Inflammatory Bowel Disease (IBD) by healthcare professionals, this API allows for comprehensive remote monitoring including biomarker measurements by using only one patient application. For instance, a unique application can be used to answer Quality of Life (QoL) questionnaires, messaging to healthcare professionals and perform fecal calprotectin tests at home. With all these features, a patient can be followed for a long period of time and without the need to come to the hospital if it is not needed. For the healthcare professionals, it means that all the results provided by this application are available in one spot. With the rise of many different electronic systems, there is a clear benefit of such an integration tool, avoiding healthcare professionals having to interact with multiple dashboards and tools.
One country has been pioneering the benefits of this solution for the patients and the healthcare professionals early on: Sweden. In this post, we are presenting two evaluations of the IBD Home solution. The evaluations are in Swedish and below is a summary of the original articles: Capio St. Goran hospital evaluation and Västra Götaland region evaluation.
Back in 2017, Telia Company, a Swedish telecommunication provider, created an integrated solution for the patients in partnership with BÜHLMANN: an application allowing them to answer quality of life questionnaires, doing fecal calprotectin tests at home using IBDoc® and an easy logistic system having IBDoc® tests available at the nearest possible pharmacy. This solution, called IBD Home, allows the results to be shared with the healthcare professional through SWIBREG, the national quality register for IBD. This national program is in use all over the country and is available to any IBD patient in Sweden.
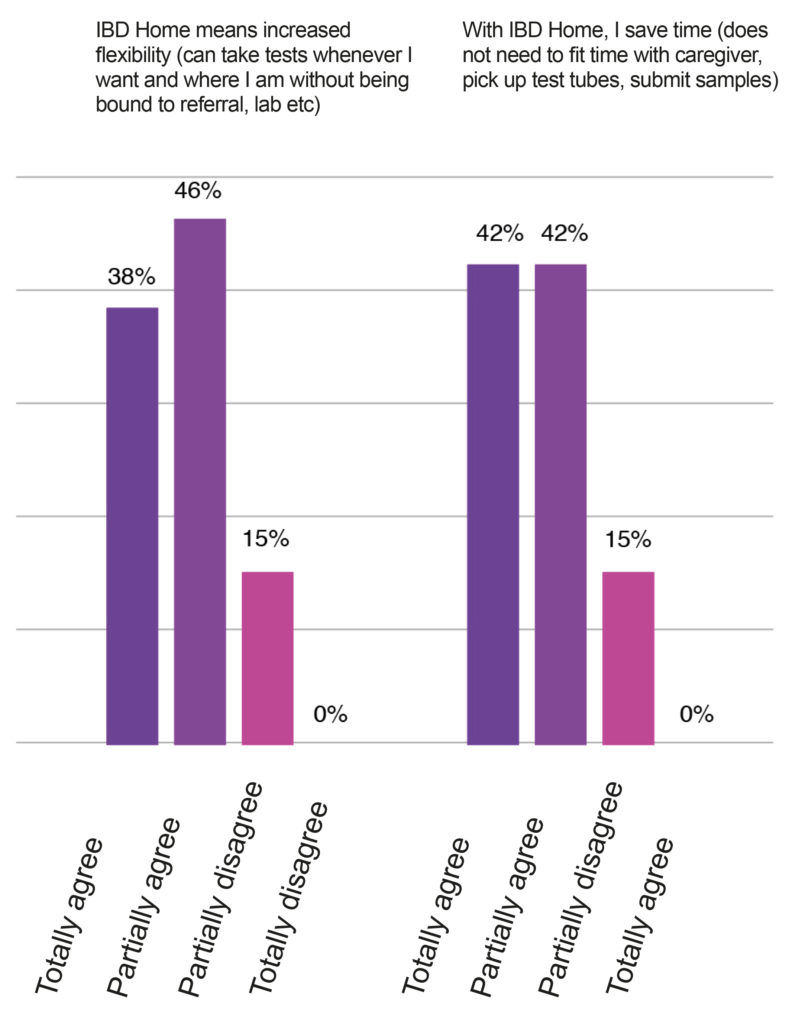
In 2020, the Capio St. Goran hospital wanted to implement this solution in their gastroenterology department. Within a few months, they were able to recruit about 70 patients to the program and completed an evaluation using surveys for the patients and the healthcare professionals. The response from patients was unequivocally positive. For most of them, IBD Home provides increased flexibility and security as they do not have to go to the lab and get their results immediately (Figure 1). After a few months of usage, 80% of the patients would recommend IBD Home to other patients in their situation.
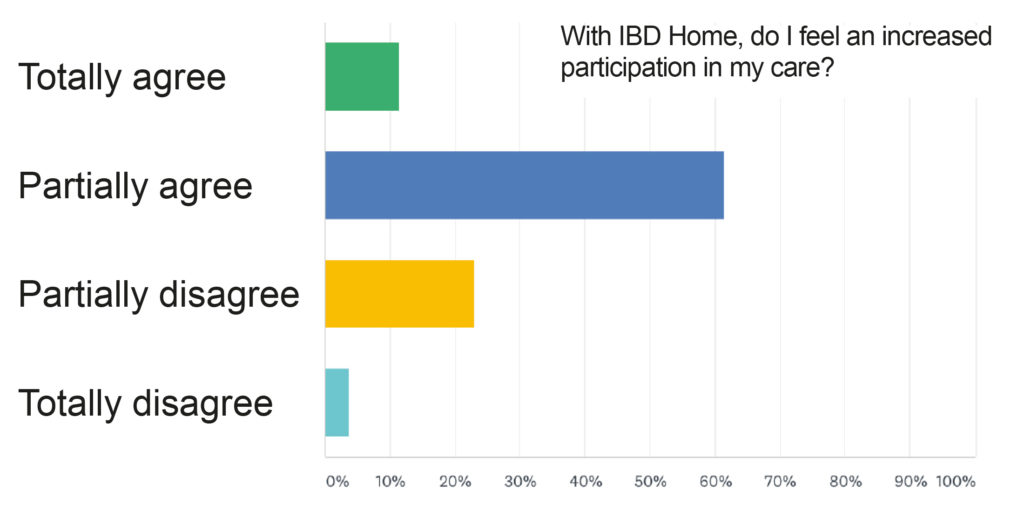
In addition, the IBD nurse Birgitta Håkansson, participating in the program, stated that she felt that patients were more involved in their care (Figure 2). This resulted in improved conditions for the healthcare staff as well. Because more patients want and can take a larger own responsibility, the care staff can reduce the number phone calls and instead can focus on the patients which are actually in need for their resources.
In addition to this hospital study of the IBD Home program, there was an official evaluation done by the Västra Götaland region within the framework of restructuring the healthcare and program area in digitization. Since June 2017, the IBD Home program was offered to IBD patients and it was found that in this region, 338 patients were included. To measure the usefulness of the solution, patients were asked to take surveys at 3, 6 and 12 months. The results were excellent, and patients were satisfied with the service. It was noted that the main advantage for the patients was not to bring their stool samples to the lab, offering them more flexibility and time. Surveys were also given to the healthcare professionals at 3, 6 and 12 months and the results were positive too. The majority of them think that including patients into the program is not time consuming and is even saving time as it is allowing them to free up time to focus on patients that are actually in need of medical attention while other matters can be resolved by telephone. From a qualitative point of view, the report concludes very clearly that IBD Home creates clear patient benefits, mainly in the direction of quality of life improvements.
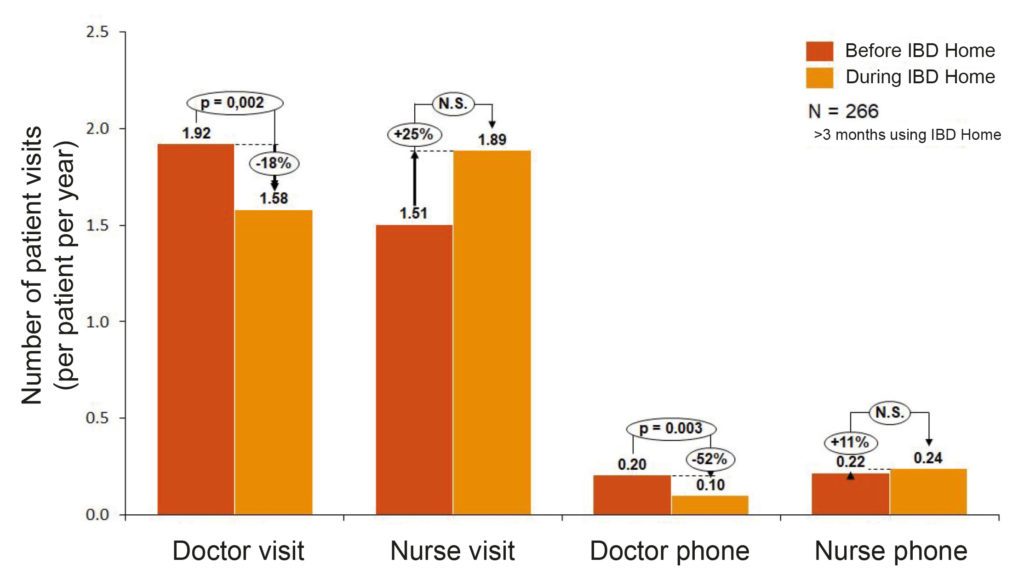
The other interesting part of this report is that they assessed IBD Home from a quantitative point of view, looking at patient visits and costs. It was found that, with IBD Home, the number of doctor visits fell by an average of 18 percent with a small increase of nurse visits, which indicates a redistribution from doctor visits to nurse visits. On the same note, emergency admissions decreased by 32 percent during year one and 44 percent during year two for the entire region. The reduction in the number of doctor visits could be explained by the fact that IBD Home creates opportunities for self-care as well as self-control of the patient’s disease, which leads to patients being able to earlier identify possible relapses and thereby implement preventive measures together with care. A proactive follow-up and control of any relapses also reduces the need of emergency admissions to hospitals. Based on available data, IBD Home means a saving of about SEK 400 (~40€) per year and patient. In addition, IBD Home can contribute to increased precision in drug treatment, which can result in both financial and patient-related benefits.
If you want to know more about our API program, now including HL7/FHIR communication, please contact us at support@ibdoc.net.
- Heida A. et al., 2017, Agreement Between Home-based Measurement of Stool Calprotectin and ELISA Results for Monitoring Inflammatory Bowel Disease Activity, Clin Gastroenterology and Hepatology.
- Walsh A. et al., 2018, Defining Faecal Calprotectin Thresholds as a Surrogate for Endoscopic and Histological Disease Activity in Ulcerative Colitis—a Prospective Analysis, Journal of Crohn’s and Colitis.
- Haisma et al., 2019, Head-to-head comparison of three stool calprotectin tests for home use, Plos One.