
An interview with Prof. Dr. Patrick Van Rheenen
University Medical Center Groningen, The Netherlands
Can you introduce yourself and your organization?
My name is Patrick van Rheenen and work at the University Medical Centre in Groningen (the Netherlands). I am a clinical researcher into paediatric inflammatory bowel disease.
How did you choose IBDoc® for the Flarometer project?
Part of my research focusses on home telemonitoring. We showed that reducing the number of face-to-face health checks and building in calprotectin-based telemonitoring was equally safe for children as conventional face-to-face health checks. That time we used sent-in stool samples, and we found that the quality of the sample deteriorates on its way to the lab. This was especially the case when the stool sample pack was dropped in a postbox in summertime, with prolonged exposure to high temperatures. To overcome this problem, we wanted patients to test their fecal calprotectin level at home. And that is where IBDoc® came in. We chose IBDoc® after a head-to-head comparison of the different solutions on the market1. With IBDoc® showing a good comparison to the laboratory results and the lowest number of reading errors, it gave us the confidence to use this system.
Can you describe the Flarometer project?
The word Flarometer is derived from barometer, which is a device that measures changes in air pressure and predicts the weather. With the Flarometer, we measure changes in disease activity and predict a flare. The Flarometer score is composed of two elements: the calprotectin home test and the disease activity score (Figure 1). We wanted to move our telemonitoring strategy from a demonstration project to one that is sustained within our hospital. For that purpose we made the following necessary adaptations:
• Integration of IBDoc® data in our electronic patient file (EPIC).
• Easy processing of order placement and invoicing via EPIC and the laboratory information system (GLIMS).
• Sending automated email reminders and disease activity questionnaires through the patient portal myUMCG.
What made you want to integrate IBDoc® into the Flarometer?
In our hospital we already used BÜHLMANN‘s calprotectin assays. We wanted a home test that could be used interchangeably, and IBDoc® best suited this task. And BÜHLMANN was willing to invest in an interface based on the HL7 FHIR standard that allows a system that safely connects various kinds of medical databases.
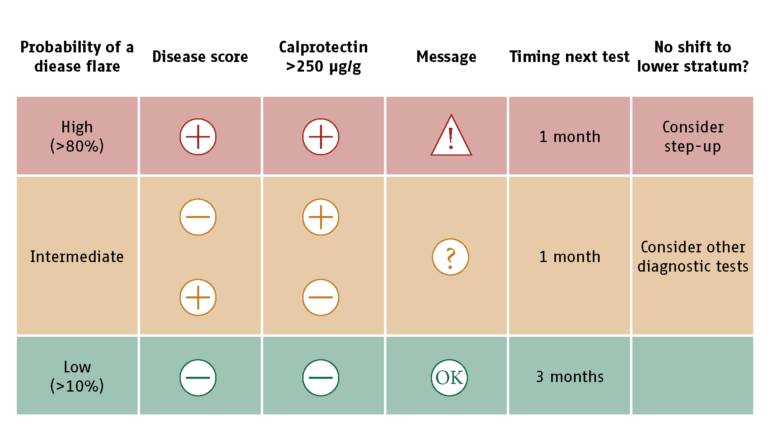
Figure 1: Flarometer strategy. Algorithm with advice on treatment and the timing of re-measurement2
Who is administrating Flarometer? The clinicians or IBD nurses?
Technical competence in performing a diagnostic test is not only important for laboratory staff, but also for patients who wish to use the home test. Patients should be trained before they can start with home measurements. The training is given by our nurse practitioner, who also manages the telemonitoring service. It takes her about 20 minutes to train the children and their parents and to register them in the Flarometer system. This is time well spent as it will reduce the number of future face-to-face checks. When the nurse places an order for a calprotectin home test, the hospital laboratory sends a test kit to the patient’s home (Figure 2). The patient will then wait for an automated prompt via the myUMCG app to test the fecal calprotectin with IBDoc®. The test result is stored at the IBDoc® portal and is pulled into the laboratory information system. The laboratory staff reviews the result before it is released to the electronic patient file. The Flarometer system does not take fully automated decisions. The clinical staff interprets the results and decides on the next appropriate action. A disease activity score and the IBDoc® result cumulate in a relapse risk stratification (Figure 1). In brief, a patient in the low-risk stratum (symptom score below 10 and IBDoc® result below 250 μg/g) will be reassured through the myUMCG app and advised to retest in 3 months. A symptomatic patient with an IBDoc® result above 250 μg/g is considered to have active disease and is fast-tracked for an appointment with the IBD team. A patient in the intermediaterisk stratum (normal disease activity score and elevated IBDoc® result, or vice versa) is contacted via phone by the IBD nurse and will have to repeat the assessments in a month time (see also Figure 2).
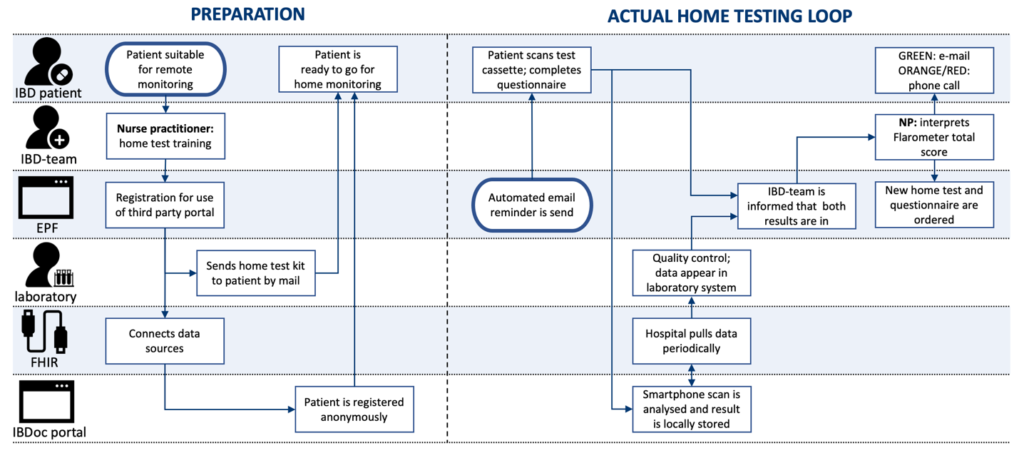
Figure 2: Flarometer care pathway
How do your patients welcome this remote monitoring solution?
Patients who participate in our telemonitoring service get an instant Flarometer score that comes with an advice. A low score indicates disease remission and the patient can continue his or her life without a face-to-face health check. A high Flarometer score indicates a flare for which an adjustment of the therapy plan is required. The patient can then make an appointment for our rapid access clinic. Our motto is: “Stay at home if you can, come to the hospital if you have to.” The Flarometer system is not meant to fully replace the face-to-face checks but should be considered as an addon to conventional health visits. As part of our Standard of Care we see our stable paediatric patients every 6 months.
Since the start of the project, how would you assess its effectiveness?
At this moment, about a third of our 180 patients (and their parents) use the telemonitoring service, and the proportion is gradually growing. Every patient with newly diagnosed IBD is now immediately trained in calprotectin home measurements as a Standard of Care, just like new diabetes patients are trained in glucose measurements.
Are the patients compliant with the tests?
Some patients seek as much information as possible about the threat for a disease flare (‘monitors’), while others try to avoid potentially threatening information (‘blunters’). I do not need to explain which type of patient is more suitable for remote monitoring. With this said, the majority of my patients fall into the “monitors” category, and we see a compliance rate of 85 %. For the 15% of patients that do not respond in time, our IBD nurse practitioner will send a short personal reminder. Most patients are willing to perform the home test because they get the results instantly.
Do you plan to integrate other remote monitoring systems into the project?
Remote Therapeutic Drug Monitoring (TDM) and CRP measurements are next to come, which is particularly useful for patients on subcutaneous biologicals and for those with home infusions.
Professor van Rheenen, thank you very much for this interview.
1. Haisma S et al., 2019, Head-to-head comparison of three stool calprotectin tests for home use, PLoS One
2. Heida A, 2018, Efficacy of Home Telemonitoring versus Conventional Follow-up: A Randomized Controlled Trial among Teenagers with Inflammatory Bowel Disease, Journal of Crohn’s and Colitis