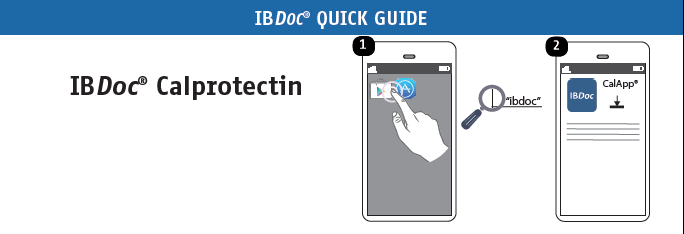
3 easy steps
to monitor IBD
IBDoc® turns your smartphone into a medical device to provide an accurate result of your fecal calprotectin.
By loading the video, you agree to YouTube's privacy policy.
Learn more
3 easy steps
to monitor IBD
IBDoc® turns your smartphone into a medical device to provide an accurate result of your fecal calprotectin.

By loading the video, you agree to YouTube's privacy policy.
Learn more
Smartphone List
IBDoc® is available on more than 90 validated smartphones. Check our list to see if yours can work with the app.
Measure your fecal calprotectin from
the comfort of your home
Take sample
Using the CALEX® Valve stool preparation device, safely take a sample of your stool and put it back in the tube to extract your calprotectin.
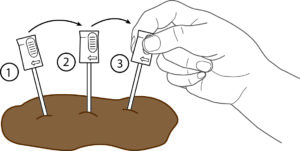
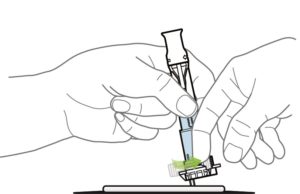
Load sample
Turn the throttle of the CALEX® Valve to easily release the liquid from the tube and let it migrate through the test cassette.
Load sample

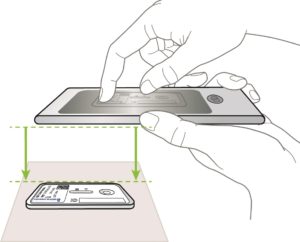
Scan test
The IBDoc® application turns the camera of your smartphone into a medical device capable of providing you with a quantitative result of fecal calprotectin with a simple scan.

I do strongly believe it should be a standard of care that all patients have access to IBDoc®.
Kathleen Sugrue