Fecal calprotectin (fCAL) is a biomarker used for disease monitoring in patients suffering from intestinal bowel disease (IBD) since it correlates closely with mucosal healing. Calprotectin measuring requires patients to collect and return stool samples resulting in relatively low return rate of samples. Home-based fCAL testing may improve adherence. In this post three recent publications from studies using the BÜHLMANN fCAL home-test IBDoc® are summarized. The publications range from usability studies, correlation to established laboratory methods to correlation to clinical performance:
Walsh et al.:
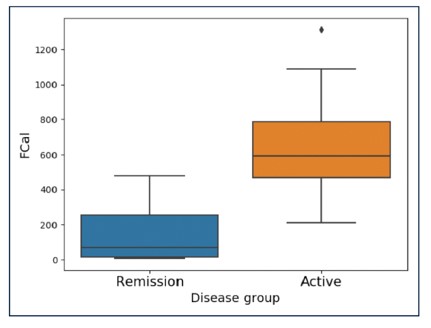
Background: The aim of this study was to correlate fCAL measured using IBDoc® with validated endoscopic and histological disease activity indices in UC.
Methods: Sixty-six patients diagnosed with UC were recruited, and data about various indices such as symptoms (Simple Colitis Clinical Activity Index, SCCAI), quality of life, endoscopic (Ulcerative Colitis Endoscopic Index of Severity, UCEIS) and histological activity (Nancy index) were collected over 6 months. Symptoms were reported daily, quality of life fortnightly, outcomes every 3 months, and endoscopy as well as biopsy at time point 0 and after 6 months. Indices were evaluated and compared to each other.
Results: There was a strong correlation between fCAL and endoscopy and an even stronger correlation between fCAL and histology. It was shown that fCAL levels <187 µg/g were not associated with active endoscopic disease with an area under the curve of 0.915. Further, FC levels < 72 µg/g were not associated with histological inflammation in UC patients with an area under the curve of 0.824.
Conclusion: For measuring disease activity of UC, as well as for endoscopic or histological remission, fCAL is a useful surrogate marker with a strong correlation of R=0.88 between IBDoc® fCAL values and histological score.
- “This is the first prospective study to use validated endoscopic and histologic indices to determine the thresholds of fCAL that have the greatest sensitivity for detecting active disease.”
- “An fCAL level <187 µg/g is not associated with active endoscopic disease (UCEIS ≥4) and an FC value <72 ≤g/g is not associated with histological inflammation (Nancy ≥2).”
- “Using these thresholds in clinical practice may help to avoid endoscopic procedures for those patients not having active endoscopic disease (FC levels <187 µg/g).”
Moore et al.:
Background: The aim of the study was to evaluate the usability of the home-based fCAL test IBDoc® and to compare the results generated by IBD patients to a lab-based ELISA fCAL test.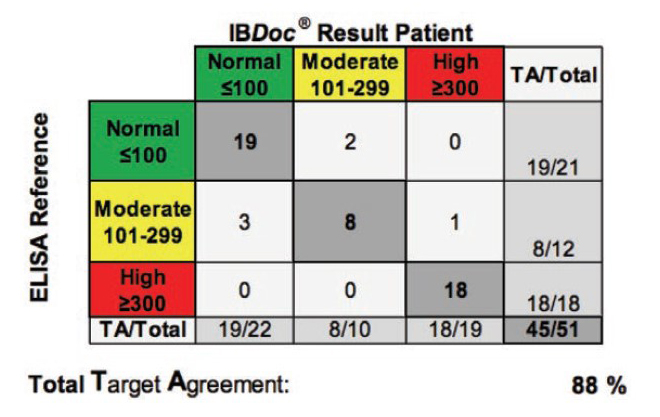
Methods: Patients suffering from CD or UC were prospectively recruited from three tertiary sites across Canada. Thereby, patients performed fCAL measuring using IBDoc® and completed a questionnaire to report the ease-of-use of the home-based fCAL testing. In addition, the BÜHLMANN fCAL® ELISA was performed using the same stool sample in order to compare home-based to laboratory-based fCAL testing.
Results: Sixty-one patients (29 CD, 32 UC), with 19 of them (25%) having active disease, were enrolled in this study. Seventy-nine percent (48 of 61) of participants agreed that the IBDoc® was easy to use and eighty-five percent (52 of 61) of the patients were willing to to use the home-based fCAL test in the future. Comparing IBDoc® results with a laboratory-based ELISA showed an 89% agreement across all values without any false positives or negatives.
Conclusion: Patients suffering from IBD found the IBDoc® fCAL home-test user friendly and results correlated well with the standard laboratory-based ELISA. Therefore, IBDoc® could enable clinicians to adopt a treat-to-target approach more easily and improve patient’s quality of life.
- “This study showed a good correlation between the fCAL measurements from the IBDoc® and the ELISA method, with no false positives or negatives”
- “The home-based fCAL monitoring kit IBDoc® is acceptable to patients to use”
- “IBDoc® enable clinicians to more easily adopt a treat-to-target approach, improve long-term outcomes and patients quality of life with IBD”
Wei et al.:
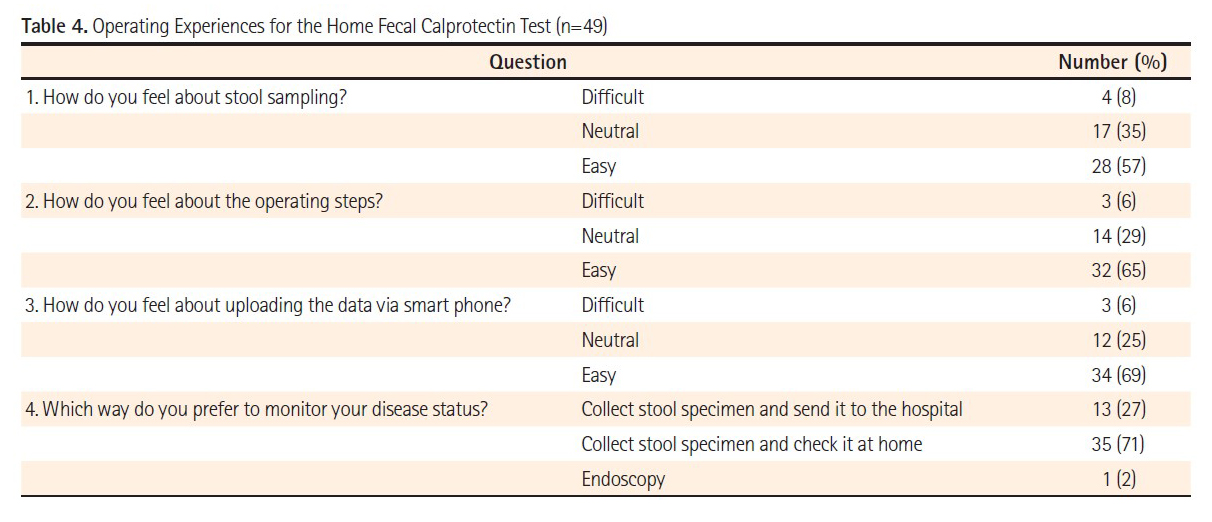
Background: The aim of this study was to evaluate the performance of IBD patients using the fCAL home test IBDoc® by comparing the results with the traditional laboratory-based Quantum Blue® fCAL. Experiences and feedback from patients using IBDoc® were observed trough questionnaires.
Methods: IBD patients in clinical remission willing to perform the IBDoc® were enrolled prospectively. Fecal calprotectin was determined by the patients itself using IBDoc® and by a laboratory based test, both using the same fecal sample. In addition, patients filled in a questionnaire about the ease of use of IBDoc®.
Results: Fifty-one patients were enrolled in this study with 27 of them diagnosed with UC, 23 with CD and one with indeterminate IBD. After use, a majority of the patients (65%) found IBDoc® easy to perform and 80% indicated a strong probability to use it for future monitoring when having an acceptable price. Only two patients (4%) felt that IBDoc® was difficult to manage. Seventy percent of the IBD patients preferred to monitor their disease activity with the home-test rather than sending them to the laboratory or perform endoscopy. The correlation between IBDoc® and laboratory-based test results was very good.
Conclusion: The correlation between calprotectin results observed with the IBDoc® and the Quantum Blue® fCAL was very good. Most patients preferred the home test over the laboratory test and endoscopy, since it was easy to perform. This enables IBDoc® to get used as an objective patient-reported outcome tool for IBD patients.
- “96% of the patients were satisfied with the home test”
- “Most patients found the home test to be feasible and easy to use and preferred it over laboratory test and endoscopy for monitoring”
- “The correlation between home fCAL test, which is handled by the patients themselves, and the laboratory test, which is performed by trained staff was very good”
References:
- Walsh A. et al., 2018, Defining Faecal Calprotectin Thresholds as a Surrogate for Endoscopic and Histological Disease Activity in Ulcerative Colitis—a Prospective Analysis, Journal of Crohn’s and Colitis.
- Moore AC. et al., 2018, IBDoc® Canadian User Performance Evaluation, Inflammatory Bowel Diseases.
- Wei S. et al., 2018, Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self-monitoring, Intestinal Research.